Abstract
Background: Despite recent advances, multiple myeloma (MM) remains incurable and becomes refractory to immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs) and anti-CD38 monoclonal antibodies (anti-CD38 mAbs). The prognosis of patients with triple-class refractory MM (TCR MM) is poor because of limited alternate therapies. The increasing number of patients with TCR MM leaves an unmet need for effective treatments in this population. The oral exportin-1 inhibitor selinexor has a novel mechanism of action by blocking nuclear export, which leads to accumulation of tumor suppressor proteins in the nucleus and blockage of protein translation of oncogenes. Selinexor is indicated for treatment of relapsed/refractory MM (RRMM) in combination with either dexamethasone (Xd) or bortezomib and dexamethasone (XVd). Approval of the QW regimen of selinexor, bortezomib and dexamethasone (XVd) was based on the BOSTON study (NCT03110562), in which 195 patients receiving XVd had a median progression free survival (mPFS) of 13.93 months vs 9.46 months in 207 patients who received twice-weekly Vd. Results from previous studies in RRMM (STOMP phase 1/2 Study NCT02343042; phase 1 Study NCT02199665) confirmed that a combination of QW Xd with another PI, carfilzomib, is associated with impressive efficacy, an overall response rate (ORR) of 78%, including in patients with TCR MM (ORR = 67%) and patients with carfilzomib-refractory MM (ORR = 62%). The long mPFS, extending over a year, including in TCR MM, indicates selinexor-containing regimens provide prolonged disease control with good tolerability.
Based on those results, we investigated XKd in a subset of STOMP patients with TCR MM with the aim of further exploring the tolerability of XKd in this patient population.
Methods: The STOMP study XKd arm enrolled patients ≥18 years of age with RRMM. Patients were administered the QW combination of oral selinexor (60-100 mg), dexamethasone 40 mg, and IV carfilzomib 56-70 mg/m2. Treatment-emergent adverse events (TEAEs) were used to assess safety and tolerability. Efficacy endpoints included ORR, duration of response (DOR), PFS, overall survival (OS), and time to response (TTR).
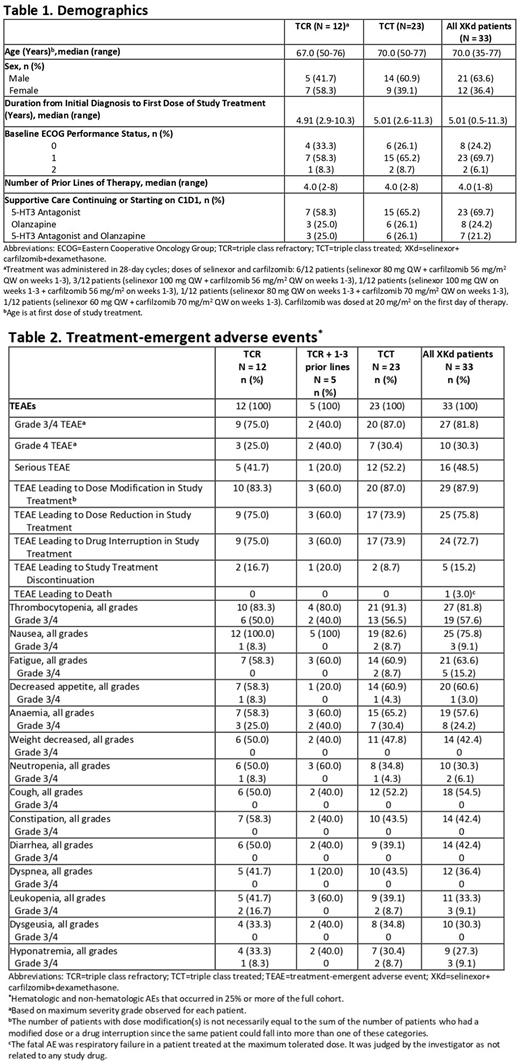
Results: Of the 33 patients treated with XKd, 23 had triple-class treated (TCT) MM. Twelve had TCR MM, including 8 (66.7%) with high-risk cytogenetics del(17p), t(4;14), t(14;16) and/or gain 1q. The median age of patients with TCR MM was 67 and the median number of previous lines of therapy was 4 (range, 2-8) (Table 1).
The rate of TEAEs in the patients with TCR MM was comparable to rates in the full cohort and the TCT subgroup (Table 2). Common hematologic TEAEs included thrombocytopenia (83.3%) and anemia (58.3%). Non-hematologic AEs included nausea (100%), decreased appetite (58.3%), and fatigue (58.3%). All cases of fatigue and all but a single event of nausea and decreased appetite had a severity grade of ≤2, and these low grade TEAEs were largely reversible and manageable with supportive care and dose modifications. Two patients discontinued study treatment after 149 and 386 days due to TEAEs, specifically cardiac toxicity attributed to carfilzomib (56 mg/m2 QW). There were no deaths due to TEAEs.
The ORR was 66.7% (95% CI, 34.9-90.1), including 5 (41.7%) very good partial responses and 1 (8.3%) complete response. Median DOR was 12.0 months (95% CI, 12.0-not estimable [NE]). Median PFS was 13.8 months (95% CI, 5.3-NE) and median OS was 33 months (95% CI, 20.4-NE). Median TTR was 1 month (95% CI, 0.9-NE). In TCT MM, results were similar with an ORR of 65.2% and median PFS of 14.3 months (95% CI 12.0-NE).
Tolerability is evidenced by the duration of study treatment of more than 6 months in 58.3% of patients and median time to discontinuation of 7.1 months (95% CI 4.6-NE). The median weekly selinexor dose was 60.8 mg.
Conclusions: Despite a small sample size, these results highlight the potential utility of XKd as a treatment regimen for patients with TCR MM. XKd was generally well tolerated in this patient population with a generally manageable AE profile and a median DOR of 12 months. These results compare favorably to that found in the MAMMOTH study, where patients previously treated with anti-CD38 mAbs who primarily had TCR MM experienced an ORR of 31% and mPFS of 3.4 months when treated with standard treatment regimens. Interestingly, in MAMMOTH, the best ORR (47%) and mPFS (5.7 months) were observed in patients treated with carfilzomib-alkylator combinations.
Disclosures
Schiller:Pfizer: Research Funding; Arog: Research Funding; Geron: Research Funding; Deltafly: Research Funding; Cellerant: Research Funding; Mateon: Research Funding; Medimmune: Research Funding; Incyte: Other: speaker fees, Research Funding, Speakers Bureau; Regimmune: Research Funding; Novartis: Honoraria, Other: Speaker fees, Research Funding; Ono Pharma: Honoraria; AstraZeneca: Honoraria; Sellas: Research Funding; CTI: Research Funding; Daiichi-Sankyo: Research Funding; Millennium: Research Funding; Deciphera: Research Funding; PreCOG LLC: Research Funding; Forma: Research Funding; Kite, a Gilead Company: Research Funding, Speakers Bureau; Jazz: Consultancy; Actuate: Research Funding; Constellation: Research Funding; Cyclacel: Research Funding; Celgene: Consultancy, Research Funding, Speakers Bureau; Glycomimetics: Research Funding; Karyopharm: Research Funding, Speakers Bureau; Gilead: Research Funding; Stemline: Speakers Bureau; Cellectis: Research Funding; Gamida: Research Funding; Bristol Myers Squibb: Current equity holder in publicly-traded company, Speakers Bureau; FujiFilm: Research Funding; Genentech-Roche: Research Funding; Amgen: Current equity holder in publicly-traded company, Honoraria; Johnson & Johnson: Current equity holder in publicly-traded company; Stemline: Research Funding; AltruBio: Research Funding; Trovagen: Research Funding; AVM Biopharma: Research Funding; Samus: Research Funding; Janssen: Research Funding; Astellas: Research Funding, Speakers Bureau; Agios: Consultancy, Honoraria; Actinium: Research Funding; AbbVie: Research Funding, Speakers Bureau; Onconova: Research Funding; Sangamo: Research Funding; Takeda: Research Funding; Tolero: Research Funding. Tuchman:Shattuck Labs: Honoraria; Prothena: Honoraria; Janssen: Honoraria. Baljevic:BMS/Celgene, Cardinal Health, Sanofi-Genzyme, Oncopeptides, Janssen, Karyopharm, NCCN, CurioScience, AJH: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Rossi:BMS, Sanofi, GSK: Consultancy. Kotb:Pfizer: Honoraria; Merck: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Takeda: Honoraria; Janssen: Honoraria; Amgen: Honoraria; BMS: Honoraria; Karyopharm: Current equity holder in private company; Celgene: Honoraria; Akcea: Honoraria. White:BMS/Celgene: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Forus: Consultancy, Honoraria; Antengene: Consultancy, Honoraria. Bahlis:AbbVie, Amgen, Bristol Myers Squibb, Celgene, Forus, Janssen, Genentech, GSK, Karyopharm, Novartis, Pfizer, Takeda, Sanofi: Consultancy; Pfizer: Research Funding. Sutherland:Janssen: Consultancy, Research Funding; Karyopharm: Research Funding; GSK: Research Funding; Amgen: Consultancy; Celgene: Consultancy; Sanofi: Consultancy. Madan:Janssen, Amgen, GSK, BMS, Karyopharm, Oncopeptide, Pfizer: Honoraria, Speakers Bureau. Leblanc:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees. Venner:Janssen, BMS, FORUS, Abbvie, Takeda, Sanofi, Pfizer, GSK: Honoraria. Bensinger:Janssen, Amgen, BMS, Takeda: Speakers Bureau. Van Domelen:Karyopharm: Current Employment. Zhang:Karyopharm: Current Employment. Bentur:Karyopharm: Current Employment. Lipe:Seagen Inc.: Research Funding; Amgen: Research Funding; Harpoon: Research Funding; GSK: Consultancy; Sanofi: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding.
OffLabel Disclosure:
selinexor, which is FDA-approved in combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy and in combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal